Paramyxoviridae
The Paramyxoviridae family consists of two subfamilies: 325a Paramyxovirinae with the genera Paramyxovirus and Morbillivirus (mammalian only); and Pneumovirinae with the mammalian respiratory syncytial viruses and turkey rhinotracheitis virus. Members of this family have nonsegmented single-stranded RNA of negative polarity and an enveloped, helical, capsid symmetry. Virions are generally pleomorphic, rounded and 100 to 500 nm in diameter. A filamentous form 100 nm wide and variable in length has been described but may be an artifact. The virion surface is covered with 8 nm projections (so-called “herring bone”) nucleocapsids that may be released from disrupted particles. The members of the Paramyxovirus (PMV) genus have neuraminidase, which is absent in the other genus.9 Virus replication takes place entirely in the cytoplasm in accordance with the scheme employed by negative-strand RNA viruses. Virus attaches to host cells through the “HN” polypeptide of the virus. Fusion of the virus and host cell membranes takes place (mediated by the “F” protein of the virus) and the nucleocapsid enters the host cell. The “F” and “HN” proteins require cleavage by host-derived enzymes and these procedures control pathogenicity in some strains.
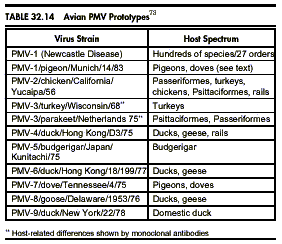
Avian Paramyxovirus
Newcastle disease virus (NDV) is the type strain for avian paramyxoviruses. Numerous, serologically different strains of this virus have been isolated world-wide. 81 Hemagglutination inhibition (HI) tests, neuraminidase inhibition tests, serum neutralization tests and comparison of structural polypeptides have resulted in the identification of nine serotypes (PMV-1 to PMV-9).4 Strains are designated according to serotype: species or type of birds from which virus was isolated/geographic location of isolation (usually country or state)/reference number or name/year of isolation.8 Table 32.14 lists the prototypes of various avian PMV serotypes. Avian PMV, particularly NDV, are important pathogens in domestic poultry and have prompted control measures that have had serious effects on international trade and movement of birds. Environmental and chemical stability, routes of transmission and pathogenesis of infections have been studied only with NDV. Comparisons with other serotypes are subjectively based.
PMV-1
PMV-1 consists of NDV and related strains that are serologically, molecular biologically and pathogenically unique. They are found in Columbiformes and some Psittaciformes. Strain-specific monoclonal antibodies are necessary to distinguish infection caused by these strains of PMV-1, which have been divided into nine distinct groups.8 Group P contains the pigeon isolates, which are no longer considered to be classic NDV.
Newcastle Disease
NDV is distributed worldwide with the possible exception of the various islands of Oceania. Birds from these islands should be considered immunologically naive with respect to NDV. NDV is serologically uniform and isolates are divided based on their virulence and epizootiologic importance (velogenic, mesogenic or lentogenic). These divisions are applicable only to the domestic chicken. Virulence is host-specific and varies considerably with experimental infections in other species.46 The host spectrum includes hundreds of species from at least 27 orders.195 Susceptibility and the clinical course of disease are highly variable between species and apparently depend on the epitopes and the enzymatic status of the host. Birds of all ages are susceptible to infection. Although overheating may be a triggering factor, no real seasonal peaks have been described. Table 32.15 shows the susceptibility of a variety of orders.91,148 Some mammals are susceptible to NDV, and humans may develop a severe conjunctivitis.
Transmission
Virus enters the host mainly through the respiratory and gastrointestinal tracts. Embryos can be infected if their shells are contaminated with virus. Vertical transmission can occur, but is rare with velogenic strains because viremic hens usually stop laying. Lentogenic and apathogenic NDV might be egg transmitted via the vitelline membrane. This route of transmission is thought to occur regularly following vaccination with live lentogenic strains (Hitchner B1). Although virus can be found in respiratory secretions, the main route of viral shedding is the feces. Mechanical vectors that may spread the virus include wind, insects, equipment and humans. Immune birds can function as carriers and intermittently shed virus. Persistent infections are limited to weeks or months. The most common carriers (reservoirs) include free-ranging waterfowl, Pittidae, Psittaciformes, some Passeriformes and Strigiformes. 21,45,59,90,157,158,183, 249,269,296,354,366,394
Pathogenesis
NDV has an affinity for erythrocytes allowing the virus to be widely distributed throughout the host’s body. Dyspnea may be caused by lung congestion and damage to the respiratory center. Petechiation results from viral adherence and damage to the vascular endothelium. The highly variable virulence of a given strain in a particular host is governed by the amino acid sequences of the “F” and “HN” viral proteins and the type of proteases available in the host for cleavage of the protein precursors.13 The incubation period varies depending on the host species, previous virus exposure, pathotype of virus and titer of infecting virus.
Clinical Disease and Pathology
Lentogenic, mesogenic and velogenic strains of NDV produce varying clinical disease in chickens. The clinical expression varies widely in other birds, even between two species of the same genus. Several clinical presentations are characteristic, but may vary considerably in their severity. In short, these can be summarized as follows:

- Peracute death; several hours of depression caused by viremia.
- Acute gastrointestinal disease (VVND); voluminous greenish diarrhea accompanied by anorexia, lethargy and cyanosis.
- Acute respiratory disease; upper respiratory exudates, rales and dyspnea.
- Acute gastrointestinal and respiratory disease.
- Chronic central nervous system (CNS) disease characterized by opisthotonos, torticollis, tremors and clonic-tonic paralysis of the limbs.
For the rule-out
list, infectious and noninfectious causes of gastrointestinal or respiratory
tract disease should be considered. One differentiating factor is that
ND is not associated with sinusitis. CNS lesions are typical for ND in
a variety of bird species. As a rule, the incubation time is prolonged
in these cases, and histopathologic lesions may be difficult to document.
Comparable clinical signs may be seen with chlamydiosis (meningitis),
salmonellosis (encephalitis purulenta) encephalomalacia, lead toxicity
and calcium deficiencies. Histopathologic differentiation is only possible
following thorough examination of a variety of affected tissues.
Antemortem diagnosis
of NDV can be performed by culturing virus from feces or respiratory discharge
(swabs) from affected birds. The number of samples required for a diagnosis
depends on the size of the flock, the clinical signs (CNS) and the quarantine
situation.
Feces or respiratory swabs should be placed in appropriate transport media, and any sample for virus isolation or serology should be shipped on ice (4°C). Serology results (HI or AGP) generally require two days, while culture results may take from three to five days to several weeks. Postmortem samples for virus isolation should include trachea, lung, spleen, liver and brain shipped in transport media on ice. Fixed tissues from the brain and trachea can be used for histopathology. Cryofrozen sections of the nasal or tracheal mucosa may be processed for staining with fluorescent antibodies (nonspecific reactions can occur). Fluid from the aqueous humor can be collected for HA (detect virus) and HI (antibodies to virus) and can provide the most rapid diagnosis (hours to days), if sufficient antigen is present in the sample.
- Direct Virus Demonstration: Virus isolation can be achieved using feces, cloacal swabs or discharge from the respiratory tract. Isolation of the virus is required for complete classification. The ability of NDV to adapt to a variety of host systems can make it difficult to demonstrate directly. The fact that latently infected birds have low virus titers and that vaccine strains (even mesogenic ones in imported or migratory birds) may be present, complicate the evaluation of virus isolations.
- Indirect Virus Demonstration: The response to antigens by the production of humoral antibodies varies within taxonomic groups and individually. Therefore, indirect virus demonstration by humoral antibodies may be difficult. HI titers can be present by the fourth day post-infection and may vary considerably. Titers may be nonexistent or low (birds of prey, domesticated pigeons, budgerigars), even in birds that have survived the disease. The development of HI antibodies may be delayed, and latent infections can result in the formation of antibodies. The HI titers that develop in Psittaciformes may be low with Amazon parrots and Psittaculidae, having average titers of 1:8 to 1:64, while cockatoos may have titers of 1:320.
Hyperimmune serum (2 ml/kg body weight IM) can be used to protect exposed birds but is of no benefit once clinical signs are present. CNS signs occur in the presence of humoral antibodies. Use of B vitamins and anticonvulsants for treating NDV-induced non-purulent encephalitis is discouraging; in controlled studies, there was no difference in treated or untreated groups. Following improvement (which may take a year), any disturbance or stressful event may cause a bird to have severe convulsions or tremors.111
Control
NDV occurs worldwide
and many free-ranging birds can function as carriers. Effective vaccination
regimes would be helpful in controlling infections in aviaries, breeding
farms and zoo collections; however, ND is a notifiable disease in many
countries and governmental regulations may control vaccination protocols.
Most birds in orders other than Phasianiformes must be vaccinated parenterally
for an effective antibody response to occur. Inactivated vaccines produced
for chickens are useful, provided that there are no governmental regulations
that restrict vaccination. Oil-adjuvanted vaccines have been shown to cause
abscesses surrounding the injection site in some birds and must be used
with caution. Abscesses secondary to subcutaneous infections are easier
to treat than those that occur following IM injections.111
Live vaccines produced
for chickens (and used for other Galliformes) should not be used in other
avian orders. The potential infectivity of the vaccine strain of virus
in a non-adapted host has not been determined. Vaccines administered
to Psittaciformes in the drinking water have been shown to be ineffective.
As a general consideration
in an active outbreak, emergency vaccination with Hitchner B1 and truly
apathogenic LaSota strains is possible via ocular or nasal drops (five
chicken doses per bird). These strains function as competitive inhibitors,
and the local protection induced cannot be determined by an increase in
humoral antibodies. In a recent outbreak on a farm with ornamental birds
(more than 2000 birds of more than 200 species), this vaccination method
successfully protected birds that were not yet clinically sick.65a
Dosing with live
virus vaccine followed by a booster after three weeks provides three to
four months of immunity. Inactivated vaccines provide five to seven months
of immunity. A live vaccine followed two to three weeks later by an inactivated
vaccine might provide 9 to 12 months of protection. These data are applicable
only to gallinaceous birds. Increases in HI titers following vaccination
are indicative of a host response and may not correlate with immunity.


Zoonotic Potential
Virulent poultry as well as vaccine strains of NDV can cause severe conjunctivitis in humans. Infected people usually recover with few problems.
PMV-1
A PMV-1 strain that is closely related to NDV but serologically, biochemically and pathogenically unique was first recognized in domesticated pigeons in the late 1970’s, probably having arisen in the Middle East.4,10,196 The virus reached Europe by 1981 and spread all over the world, affecting particularly racing and show pigeons.40,333 Monoclonal antibodies have shown PMV-1 pigeon strains recovered in many European countries to be fairly uniform. The host spectrum includes domesticated pigeons, feral doves and the Wood Pigeon. Sensitive (but more or less inadvertently infected) species include Cracidae, Pavoninae, Phasianinae, Common Blackbird, House Sparrow, Barn Swallow, European Kestrel, Common Buzzard, Vinaceous Amazon and Eastern Rosella.367 The virus is infectious to chickens, particularly immunocompromised individuals.333,414 Experimentally infected chickens do not become latent carriers.274 Some infections occur from ingestion of contaminated feces. Feed contaminated with pigeon or dove feces can be a source of infection for other avian species, particularly chickens.7
Clinical Disease and Pathology
Affected Columbiformes
have nondescript clinical signs including polydipsia, polyuria, anorexia,
diarrhea and vomiting.
These frequently unrecognized acute signs
are followed by clonic-tonic paralysis of the wings (more rarely the hind
limbs), head tremors and torticollis. In contrast to ND, flaccid
paresis and paralysis may occur, probably from a peripheral neuropathy.95
Other less frequent signs are unilateral blepharedema, egg deformation,
embryo mortality and dystrophic molt. Dyspnea, which is common with
ND, does not occur. Mortality is highest in nestlings. Affected older
birds may spontaneously recover within three to four weeks after the onset
of clinical signs. Gross lesions include hyperemia of the brain and
large parenchymatous organs, catarrhal enteritis, swelling of the kidneys
and hemorrhage and necrosis of the pancreas.
Histologic lesions are variable. Edema of the meninges and brain and swelling of the vascular endothelium in the meningeal vessels may be noted. Lymphocytic perivascular infiltrates and demyelination of the white matter may occur in the cerebrum, diencephalon, optic lobe, medulla oblongata, intumescentia cervicalis and lumbaris of the spinal cord. Degenerative and inflammatory lesions also occur in the peripheral nerves (plexus brachialis, plexus ischiadicus). 95 Lysis of Purkinje cells in the cerebrum, which was reported initially with PMV-1 pigeon, may have been caused by herpesvirus that was also isolated from affected birds.277
Diagnosis
Procedures designed for isolating NDV are effective for PMV-1 pigeon. The HI test can be used to differentiate between NDV and PMV-1 pigeon. Final differentiation is possible only by the use of monoclonal antibodies.
Treatment
LaSota vaccine
strains administered via eye or nasal drop are not as efficacious in protecting
from infections as expected. The LaSota strains replicate poorly in pigeon
tissue so that
high vaccine doses are necessary for interference and
antibody production (protection only for 8 to 12 weeks).170 Vaccination
with live vaccines may exacerbate latent chlamydia or pigeon
herpesvirus infections.222
Parenteral administration of live Hitchner
B1 vaccine has similar side effects but may provide six months of immunity.
Inactivated vaccines are preferable for pigeons. In an active outbreak, vaccination with an inactivated vaccine will decrease the length of the disease and mitigate the clinical signs.170 Once CNS signs develop, vaccination is of no value; however, spontaneous recoveries do occur.
Control
For vaccination,
homologous, inactivated oil emulsion vaccines are commercially available.220
Annual boosters are necessary.220 All birds in a loft, and competitive
traveling groups of homing pigeons, should be vaccinated.
Squabs from hens vaccinated three months before laying may not have protective
antibodies. 220 Squabs can be vaccinated with homologous vaccine by four
weeks of age.222 Inactivated NDV vaccines provide only six months of
protection.166
Vaccines are best applied subcutaneously in the neck. Intramuscular injections in homing pigeons can cause severe irritation of the pectoral muscles. To prevent fatal hemorrhage from the plexus subcutaneous collaris (see Chapter 44), injections must be given in the caudal third of the neck, near the middle of the dorsal aspect. Oil-emulsion adjuvants produce superior antibody titers and have fewer side effects than aqueous carbomers.48,319 An effective oral vaccine has not been developed and requires the isolation of an apathogenic PMV-1 pigeon strain.
PMV-2
PMV-2 strains that
occur worldwide display considerable antigenic and structural diversity.9
Preliminary classification using monoclonal antibodies has identified four
groups.300 Isolates from Psittaciformes, some Passeriformes, one Gadwall
as well as several turkey isolates from Israel together with a Mallard
and a Coot strain belong to group 1. Group 2 consists of chicken strains
from the Arabic Peninsula, and two strains from Passeriformes have been
assigned to group 3. In group 4, a variety of strains from Passeriformes
has been placed. The type strain PMV-2/Chicken/California/Yucaipa/56 belongs
to group 1. The host spectrum includes chickens, turkeys, Passeriformes,
Psittaciformes and more rarely, rails and ducks.
PMV-2 strains are
endemic in Passeriformes (Ploceidae, Zonotrichiinae, Zosteropidae and Estrildidae),
particularly those originating from Senegal. Isolates have been recovered
from clinically healthy imported companion and aviary birds (Estrildidae,
Viduidae, Ploceidae and Carduelidae). Experimentally, these isolates cause
a mild upper respiratory tract disease. PMV-2 infections are more severe
in Psittaciformes, particularly in African Grey Parrots, where emaciation,
weakness, pneumonia, mucoid tracheitis and mortality are common findings.64
The Bangor isolate from finches was proposed as a cause of death in a Blue
Waxbill. Experimentally the Bangor isolate caused only mild respiratory
signs and no pathologic lesions.263 Further investigations in a variety
of bird species are necessary in order to evaluate the virulence of the
various strains.
The host spectrum may be much wider than has been shown by direct virus demonstration. Antibodies against PMV-2 have been demonstrated in homing pigeons, healthy Passeriformes (many of them free-ranging) and some birds of prey. Isolates from various finches have not been shown to be pathogenic for chicks.116,204 Diagnostic methods used for PMV-1 are also applicable to PMV-2.
PMV-3
PMV-3 strains have
been isolated from chickens and turkeys in North America, Great Britain,
France and Germany. Most isolates from nondomesticated species originated
from imported Psittaciformes (lovebirds, cockatiels, budgerigars, macaws,
Psittacula spp, Neophema spp.). Some Passeriformes are also susceptible.
Antibodies to PMV-3 have not been documented in feral birds, but a free-ranging
avian reservoir probably exists.
Two groups of PMV-3 strains, one consisting mainly of turkey strains and the other of strains isolated from companion birds, can be differentiated using monoclonal antibodies.19 The virus is serologically related to NDV. PMV-3/Parakeet/Netherlands/ 449/75 will protect chickens against NDV.8 Intracerebral pathogenicity indices vary from 0.25 to 0.35 358 up to 1.3. 385
Clinical Disease and Pathology
The pathogenicity
of this virus group varies with the infected species and (probably) virus
strain. Conjunctivitis is the initial clinical sign in finches and Weaver
Finches (eg, Gouldian Finch, Red-cheeked Blue Waxbill, Canary, White-rumped
Canary, Orange- cheeked Waxbill, Black-throated Grassfinch, Double-barred
Finch and Avadavat). Yellowish diarrhea, dyspnea and dysphagia occur as
the disease progresses. Some affected birds die within a few days, while
others recover over a period of weeks.358 CNS signs are not regularly seen
in finches (Black-eared Wheater, Grey-headed Wheater, Red-breasted Flycatcher).367
Infected Psittaciformes develop CNS signs similar to those with ND. Susceptibility
in Psittaciformes is variable. African Grey Parrots may develop ocular
lesions (dilated pupils, hemorrhages around the pecten, uveitis and fibrinous
exudate into the anterior chamber), unilateral or bilateral paralyses and
hemorrhagic nasal discharge.148
Latent carriers
have been described in Siberian Rubythroat, Long-tailed Grass Finch, Nutmeg-Mannikin
and Cutthroat Finch; Japanese Quail and domesticated
pigeon.358,367,385
Detailed pathologic descriptions are not available. Liver and kidney lesions accompanied by an enteritis with blood in the intestinal lumen are common. Small birds are frequently cachectic, suggesting a chronic disease course or the inability to eat and drink. Histopathologically, hyperemia and a mild proliferation of glial cells in the brain may be seen. The typical nonpurulent encephalitis described with the CNS form of ND is not recognized with PMV-3 infections.
Diagnosis and Control
Salmonella spp., NDV, chlamydia and mycotoxins should be considered in the list of differential diagnoses. The methods for demonstration of the virus are the same as with other PMV groups. Serologically, there are cross reactions with PMV-1. An exact differentiation is possible with monoclonal antibodies. An oil emulsion vaccine was developed in Great Britain to counteract the decrease of egg production in affected turkeys. Another inactivated vaccine produced sufficient immunity in budgerigars and canaries to withstand challenge.35
PMV-5
Budgerigars are
considered the host of PMV-5. The type strain is called Kunitachi virus
289 and has been since lost. Possibly related strains have been isolated
from free-ranging Rainbow Lories and budgerigars from the same area of
Australia.284,285
Natural and experimental
infections in budgerigars are characterized by acute diarrhea, dyspnea,
torticollis and death. Affected budgerigars in Australia had severe diarrhea
with a 50% mortality rate. Affected Rainbow Lories became depressed, lethargic
and had three to four days of diarrhea followed by death. Birds were typically
anorexic but drank liberally.
Necropsy findings
in budgerigars were limited to hyperemia of the parenchymatous organs.
Rainbow Lories had swollen livers and spleens and necrotizing- to-ulcerative
or diphtheroid-to-hemorrhagic enteritis, with hemorrhages within the mucosa
of the ventriculus and proventriculus as well as edema of the intestinal
wall.
Histopathologic lesions included multiple necrotic foci in the liver and kidney with the development of giant cells. In Rainbow Lories, extensive loss of the intestinal epithelium with desquamated necrotic material and erythrocytes in the lumen was common. Mild perivascular infiltration with lymphocytes was common in edematous intestinal walls. The differential diagnosis list should include Salmonella spp., NDV, E. coli and nutritional deficiencies. PMV-5 cannot be isolated via all the same methods as other PMV strains.
PMV-7
PMV-7 has been isolated only from Columbiformes. The type strain was isolated from doves in Tennessee, and another isolate from the Rock Pigeon in Japan. All strains have a heat-stable hemagglutinin, and are considered apathogenic. Whether or not the Japanese and the New World strains are the same has not been determined.6
PMV-4, PMV-6, PMV-8 and PMV-9
These groups contain virus strains recovered from clinically healthy waterfowl located in the United States and Asia. PMV-4 is rather uniform and is apathogenic in chickens.5 The duck strains of PMV-6 may cause a mild respiratory disease and decreased egg production in turkeys.13 Isolates have been recovered by culture of tracheal and cloacal swabs.377 Details on PMV-8 and PMV-9 are limited. Carriers of PMV-4 and PMV-6 include Canada Goose, Common Teal, Common Pintail, Mallard, American Black Duck, Ring-necked Duck and Hooded Merganser.148 Parainfluenza-2-virus (PI-2-virus) The PI-2-virus, which belongs to the genus PMV, does not cause clinical disease or decrease in egg production in chickens. The virus can, however, be egg transmitted without influencing the embryonal development. The chicken PI-2-virus is identical to the agent that causes croupous pneumonia in humans. The PI-2-virus is important because it is not easily recognizable as a source of human infections and may be a contaminant in embryonated chicken eggs used for vaccine production.203,421,422
Click on one of the following underscored titles to go to that home page:
ANIMALS
| PIGEONS
| URBAN
WILDLIFE SOCIETY | AVIAN
AFFAIRS COALITION
| WILDLIFE
REHAB
ANIMAL
PROTECTION AGENCY | VETERINARY
EMERGENCY MEDICAL SERVICE
| > |